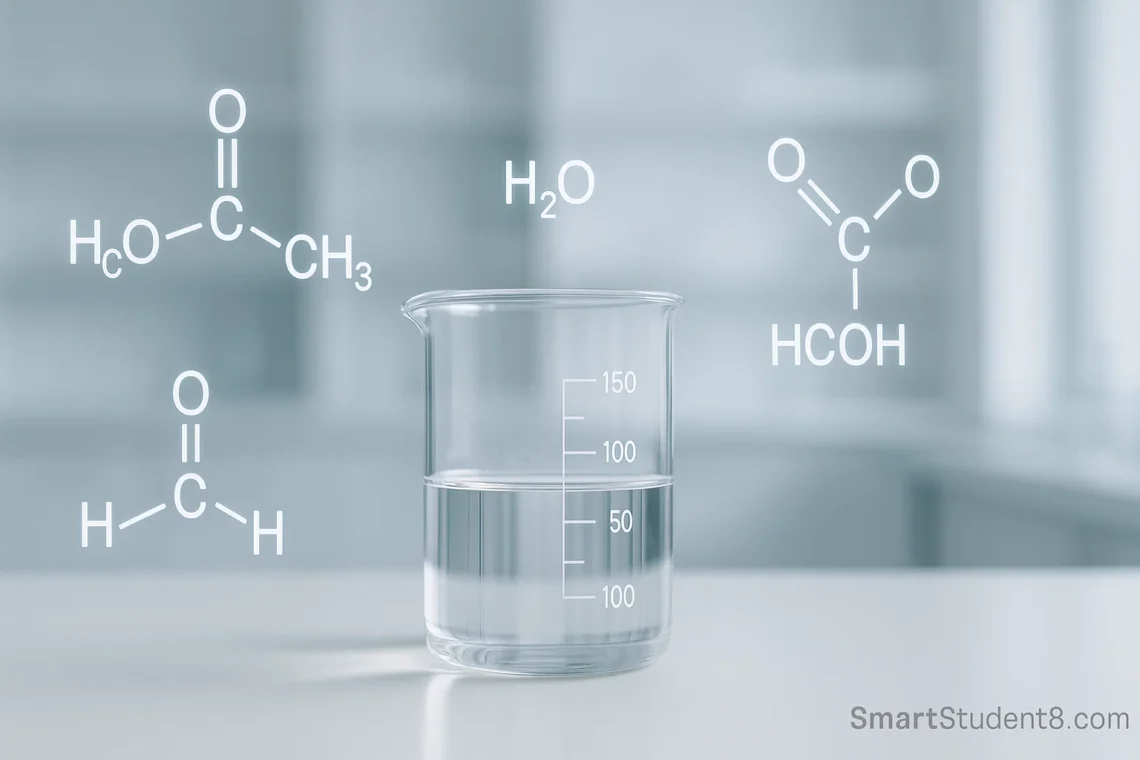
You can easily get confused by a long string of letters like “Hcooch CH2 H2O”. In reality, the formula looks complex, but isn’t a compound. This formula is actually three different components: water, methylene, and methyl formate (often written in HCOOCH3). In my experience as a chemist, who spent many years studying similar organic molecules I know how simple building block can lead to complex and useful chemical reactions. The combination of these two compounds often indicates a process fundamental in organic chemistry called ester hydrolysis. This reaction has broad implications.
This article will demystify the chemical shorthand. This article will decode each chemical component and explore their key reactions. We’ll also uncover the real-world application of these molecules. From industrial manufacturing principles to green chemistry, you’ll learn why these molecules are important.
Hcooch CH2 H2O – What’s the formula?
Let’s first address the source of confusion. It isn’t an official chemical formula. The term is a popular online typo or shorthand. This word combines methyl formate (HCOOCH3) with a methylene link (CH2) and the element water (H2O). The context of an organic reaction is crucial. The combination of these elements strongly indicates a reactive atmosphere, which is likely to be the hydrolysis methyl formate where the water breaks down the ester. Analyzing each individual part to understand its role is the first step in understanding this system.
The components explained
- Methyl Formate: Here is the star. This is an ester. It has a pleasant fruity aroma. It is the simplest ester derived by formic acid.
- (Methylene). is the building block of organic chemistry. One carbon atom is bonded with two hydrogen atoms to form a methylene. The methylene group is an important component of polymers, organic compounds and many other molecules. This could be a reference to the presence of the compound as a reaction intermediate or in related compounds.
- H2O: A universal solvent, and an important reactant. The water in this case is more than just a catalyst. It is also an active participant.
A Closer Look at Methyl Formate (HCOOCH3)
Formic acid is the methyl ester of methyl formate. It is a result of the reaction of formic (the most basic carboxylic acid found in anthrax venom), and methanol. It is an ethereal liquid that has a distinctive plum-like smell. This volatile compound was a pleasant change in smell from other chemicals, and its high flame resistance required careful handling. Because of its simple structure, it is a useful intermediate for chemical synthesis. However, it can also be used to make a variety of products, ranging from fumigants to fragrances. This ester group, which is highly reactive, makes the answer to “hcooch?ch2h2o?” so important.
Methylene Groups (CH2): What are they?
The methylene (CH2) group is a common unit in organic chemistry. The methylene group (CH2) is present in many long-chain hydrocarbons including waxes and polymers, but also in compounds such as cyclohexane. Although it does not directly take part in the basic hydrolysis, the presence of this term in your search is significant. The modularity of the organic molecules is highlighted. The CH2 group is a part of starting materials that can be used to make more complex ester molecules, or can be an element of molecules made from hydrolysis products. As an example, the organic solvent methylene-chloride (CH2Cl2) can be used. As a highly-reactive intermediate, known as carbene, it can also be found in advanced organic chemistry reaction. It is used to construct complex ring structure. In our case, it is best seen as the fundamental puzzle piece that makes up many complex organic structures.
How Water (H2O), a key component in chemical reactions, plays a pivotal role
The water in chemical reactions is often more than just an observer; it acts as the driver. When water is used in “Hcooch CH2 H2O,” it plays the part of a nucleophile. This process is called esterhydrlysis. Literally, hydrolysis is “splitting by water.” Due to the polar nature, the oxygen in water molecules has a partially negative charge. This allows it to attack the partly positive carbon atom of the ester group’s carbonyl (C=O). This attack begins a sequence of events that leads to the cleavage of the ester bonds. A base or an acid can catalyze the reaction, which speeds it up. This reaction occurs in many places, including in our bodies.
The core Reaction: Hydrolysis Methyl Formate
We now know the key players. Now let’s move on to the big event, the hydrolysis. The reaction we are describing is a classical example of nucleophilic substituted acyls. It’s a fundamental principle in organic chemistry. When the ester bonds are broken by methyl formate, it produces formic and methanol.
The chemical equation is straightforward:HCOOCH3 (Methyl Formate) + H2O (Water) – HCOOH (Formic Acid) + CH3OH (Methanol)
It is possible to reverse this reaction. Formic acid or methanol can be formed by adding a great deal of water, or removing the products when they form. A small amount of sulfuric acid or sodium hydroxide acts as a catalyser, speeding the reaction up. According to my observations, the base-catalyzed process of hydrolysis (also known as saponification) is very effective at completely breaking esters down.
Industrial Methyl Formules for Real-World Applications
The hydrolysis of methyl formate and its chemistry has many applications across a wide range industries. The versatile material is used for both industrial and specialized uses.
These are a couple of key methyl-formate applications.
- Soluble: Thanks to its outstanding ability to dissolve many compounds organic and high volatility, it’s used in the manufacture of resins and oils. The material is left behind as it evaporates.
- Fomigant and Larvicide : Because of its ability to permeate materials, as well as the toxicity it has for insects, this fumigant is effective in treating grains, crops and even soil. Pest control is made easier with this powerful tool.
- Blowing Agent: When producing polyurethane, methyl formamide is used as the blowing agent. It creates bubbles in the foaming process.
- Chemical Intermediate: Serves as a starter material to synthesize important chemicals. As an example, formic and formamide are produced using it as a primary precursor.
Methanol vs. Formic Acid: Comparison of Hydrolysis Products
The hydrolysis of methyl formate produces formic acid, and methanol. Both are important but very different chemicals. It is essential to fully understand the chemistry of this system by understanding its properties. Although both molecules are made up of simple organic atoms, they have different applications and safety profiles.
| The Feature | Formic Acid (HCOOH). | Methanol is a chemical compound that contains CH3OH. |
|---|---|---|
| Class Chemical | Carboxylic Acid | Alcohol |
| Primary Use | De-icer, preservative for livestock feed, and leather tanning | Solvents, fuel, antifreeze & chemical feedstock |
| Physical State | A colorless fuming liquid that has a strong odor | Liquid colorless and volatile with a slight, sweet smell |
| Natural Source | Bee and ant venom | Small amounts of a natural substance produced by a large number of living organisms |
| Key Hazard | Can cause eye and skin irritation | Toxic. Ingestion may cause blindness or death. |
| Industrial Role | As a rubber coagulant, and as a cleaner | The precursor for formaldehyde production, plastics and acetic acid |
This table shows that a single reaction can produce two very different products.
Safety and handling considerations
It is important to adhere strictly to the safety protocol when handling chemicals, such as methyl formate and its products. The vapors of methyl formate can create explosive air mixtures. This substance is irritating to the respiratory, eye, and skin systems. The corrosive nature of formic acid causes severe chemical burnings when it comes into contact. Methanol can be extremely toxic. Ingesting or absorption through the body of even small amounts may result in permanent blindness. Personal protective equipment is required when handling chemicals in an industrial or laboratory environment. These include safety goggles as well as chemical resistant gloves and lab coats. Work should always be performed in a well ventilated area, or even under a fumehood. This will prevent hazardous vapors from being inhaled.
Green Chemistry: The Link
Principles of green chemistry are geared towards designing products and processes to minimize or eliminate harmful substances. These principles are well aligned when it comes to the hydrolysis of ester, especially using water as solvent. As a solvent, water is non-toxic and abundant. Determining reactions, such as ester hydration, is also important for the development of biodegradable products. As an example, the popular biodegradable polylactic acid is actually a polyester. In the environment, it is broken down by hydrolysis to release non-toxic lactic acid. Chemistry can create more environmentally friendly materials and processes by studying simple systems, such as the “hcooch” ch2 h2o.
Read More: Sodiceram: The Sodium-Infused Ceramic Redefining Modern Materials
Conclusion: Pulling It Together
This term is not an official chemical designation, but serves as a way of understanding organic chemistry. The dynamic system relies on methyl formate, an important methylene-building block, as well as water. It is all centered around esterhydrolysis, the most crucial reaction. It’s been demonstrated that this reaction can break an ester down into an alcohol or an acid, both of which have important industrial applications. The chemical system that is responsible for creating foams, fragrances, and other products has far greater uses than just a curiosity in a classroom. The components of the system and their interaction are decoded to reveal the powerful and elegant logic which governs molecules.